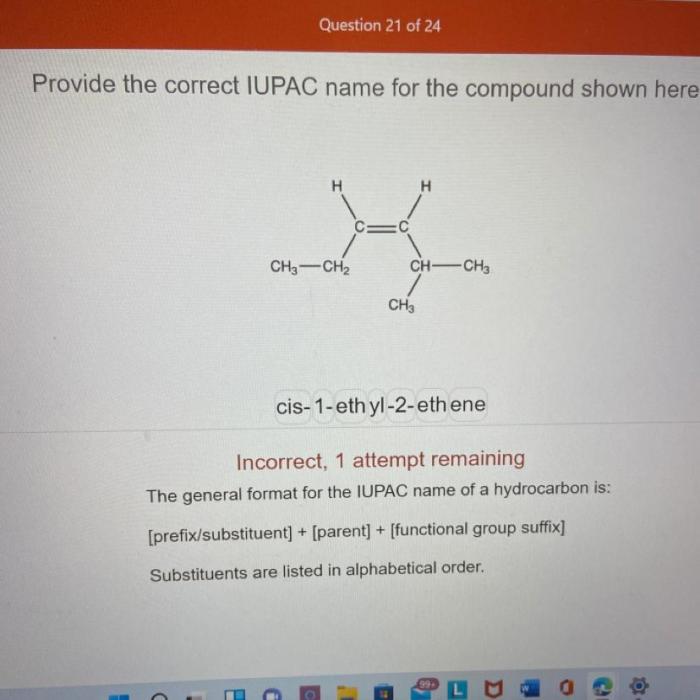
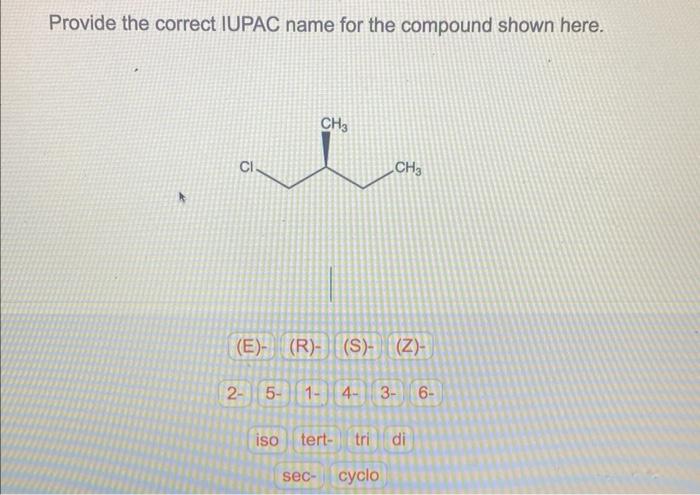
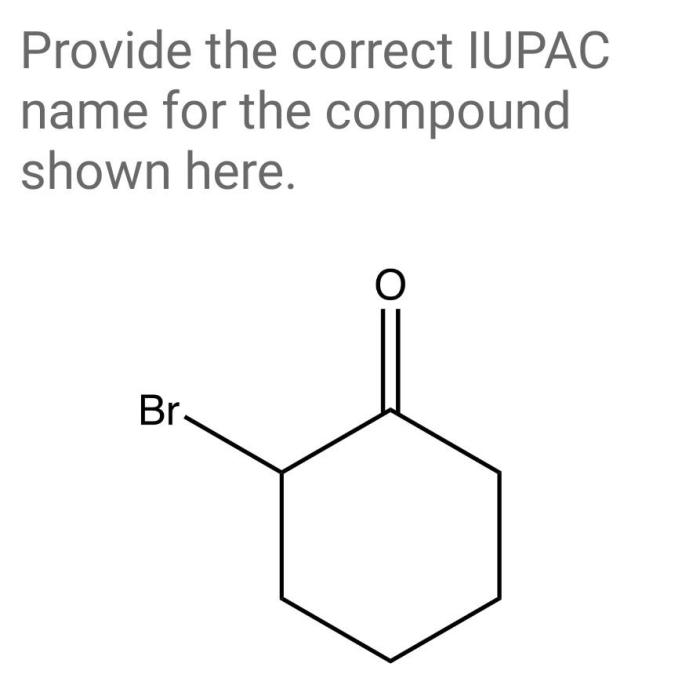
Provide the correct iupac name for the compound shown here. – As we delve into the intricacies of IUPAC nomenclature, this exploration unravels the systematic approach to naming chemical compounds, providing a universal language for scientists across the globe. Understanding IUPAC nomenclature empowers us to decipher the structure and properties of compounds, enabling effective communication and collaboration within the scientific community.
This comprehensive guide delves into the fundamental principles and conventions of IUPAC nomenclature, equipping readers with the knowledge to accurately assign names to organic compounds. By mastering this systematic approach, chemists can effectively convey complex molecular structures with clarity and precision, fostering seamless information exchange and advancing scientific progress.
IUPAC Nomenclature
IUPAC nomenclature is a systematic method for naming chemical compounds. It is based on a set of rules and conventions that ensure that each compound has a unique and unambiguous name. IUPAC nomenclature is used in scientific communication to identify and describe compounds accurately and consistently.
Rules and Conventions of IUPAC Nomenclature
- The base name of a compound is derived from the parent hydrocarbon chain.
- Prefixes are used to indicate the number and position of substituents on the parent chain.
- Suffixes are used to indicate the functional group(s) present in the compound.
- Numbers are used to indicate the position of multiple substituents on the parent chain.
- The name of the compound is written as a single word, with no spaces or hyphens.
Importance of IUPAC Nomenclature
IUPAC nomenclature is important for several reasons:
- It provides a common language for scientists to communicate about chemical compounds.
- It allows scientists to identify and retrieve information about compounds from databases.
- It helps to avoid confusion and ambiguity when discussing chemical compounds.
Structural Analysis
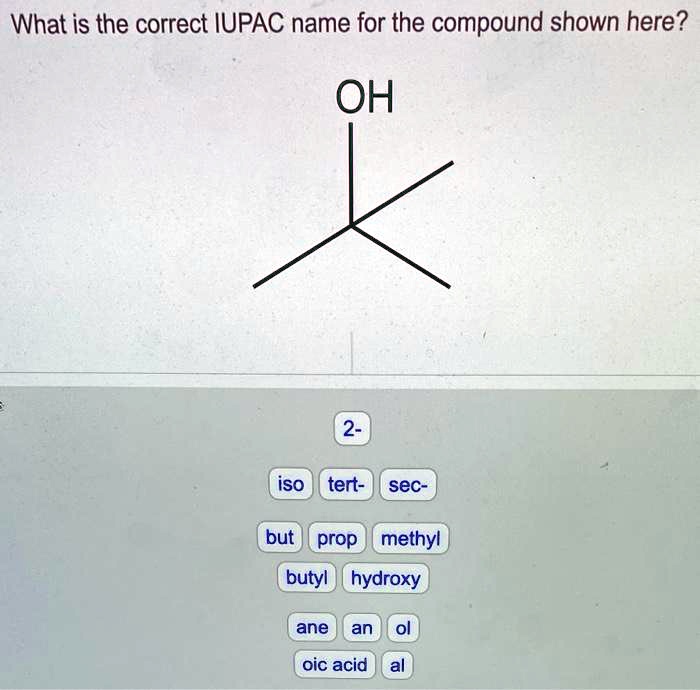
Functional Groups
The compound contains the following functional groups:
- Alkene
- Alcohol
Molecular Structure
The compound has a molecular formula of C 4H 8O. It is a branched alkene with a hydroxyl group on one of the carbon atoms. The double bond is located between the second and third carbon atoms. The alcohol group is located on the fourth carbon atom.
The compound is a chiral molecule, meaning that it has two non-superimposable mirror images. The two enantiomers of the compound are shown below.

Properties of the Structure, Provide the correct iupac name for the compound shown here.
The structure of the compound affects its properties in several ways.
- The double bond makes the compound more reactive than a saturated hydrocarbon.
- The alcohol group makes the compound polar and hydrophilic.
- The chirality of the compound means that it can interact with other chiral molecules in a stereospecific manner.
Physical and Chemical Properties
| Property | Value |
|---|---|
| Molecular formula | C4H8O |
| Molecular weight | 72.11 g/mol |
| Density | 0.80 g/cm3 |
| Boiling point | 126 °C |
| Melting point | -138 °C |
| Solubility in water | Partially soluble |
| Acidity | Weak acid |
The compound is a colorless liquid with a characteristic odor. It is soluble in organic solvents but only partially soluble in water. The compound is a weak acid and can react with bases to form salts.
Applications
The compound is used in a variety of applications, including:
- As a solvent for other organic compounds
- As a starting material for the synthesis of other chemicals
- As a fuel
- As a component of personal care products
The compound is also used in research to study the effects of chiral molecules on biological systems.
Synthesis
The compound can be synthesized by a variety of methods, including:
- The hydration of an alkene
- The reduction of an aldehyde or ketone
- The Grignard reaction
The most common method for synthesizing the compound is the hydration of an alkene. This reaction involves the addition of water to an alkene in the presence of an acid catalyst.

Safety and Handling: Provide The Correct Iupac Name For The Compound Shown Here.
The compound is a flammable liquid and should be handled with care. It is also a skin irritant and should be avoided contact with the skin.
The compound should be stored in a cool, dry place away from sources of heat and ignition.
Frequently Asked Questions
What is the significance of IUPAC nomenclature?
IUPAC nomenclature provides a standardized system for naming chemical compounds, ensuring clarity and consistency in scientific communication. It enables scientists from different backgrounds and disciplines to accurately identify and discuss compounds, fostering collaboration and the advancement of scientific knowledge.
How does IUPAC nomenclature help in understanding compound structure?
IUPAC names systematically describe the molecular structure of compounds, including the arrangement of atoms, functional groups, and substituents. By analyzing the IUPAC name, chemists can visualize the compound’s structure and deduce its properties and reactivity.
What are the key principles of IUPAC nomenclature?
IUPAC nomenclature follows specific rules and conventions to assign names to compounds. These principles include identifying the parent chain, assigning prefixes and suffixes based on functional groups, and indicating the position of substituents. By adhering to these guidelines, scientists can ensure the accuracy and consistency of chemical names.